What are the Foreign Reports and why do we include them?
Subscribe to Receive Our Weekly Call To Action by Email
Wonder has very little to say on the issue of what a nondomestic report is. From their Advisory Guide:
“VAERS occasionally receives case reports from US manufacturers that were reported to their foreign subsidiaries. Under FDA regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and unexpected (in other words, it does not appear in the product labeling), they are required to submit it to VAERS. It is important to realize that these case reports are of variable data quality and completeness, due to the many differences in country reporting practices and surveillance system quality.“
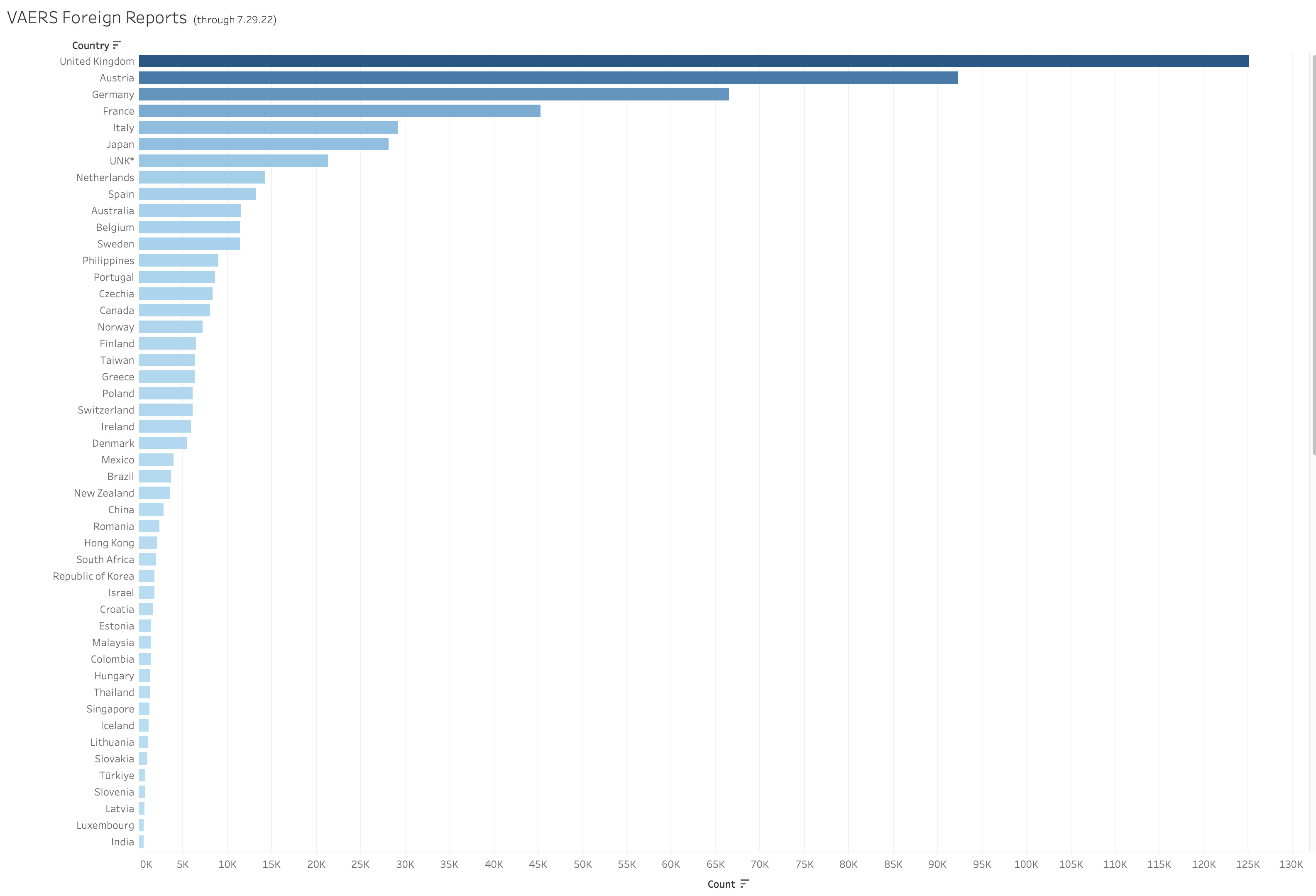
The strange thing about this is VAERS implies here that the Foreign Reports are occasional and insignificant. The numbers say otherwise. The Foreign Reports make up close to half of the reports in VAERS and more than half the deaths. For further breakdowns on Foreign Reports you can also look here: https://vaersanalysis.info/2021/07/10/4-weeks-where-foreign-vaers-deaths-reports-vastly-outnumbered-us-reports/
Here is a breakdown of the distribution of Foreign Reports as of July 29, 2022. Like the Lot Numbers these figures must be taken with a grain of salt due to the really error prone and messy nature of the data in this field*. These numbers are drawn from the data without any cleaning (this is how we do all the numbers). These are the countries with >500 reports per country code.